Automated biochemical analyzers vastly accelerate the testing of patient samples in laboratories, which has become critical now that the COVID-19 pandemic has spread across the globe. Advanced automation with motor-based motion systems will help in the fight to hasten diagnosis. Just consider the cobas 6800 and 8800 machines from Roche that automate testing of nasopharyngeal and oropharyngeal swab samples from patients suspected of having COVID-19. On March 13, 2020, the FDA issued Emergency Use Authorization for their SARS-CoV-2 tests run on cobas automated systems. This will make large-scale testing faster and more efficient.
Read more about COVID-19 testing approaches (existing an on the horizon) from our sister site: Test and Measurement Tips: How the health care system tests you for the COVID-19 virus.
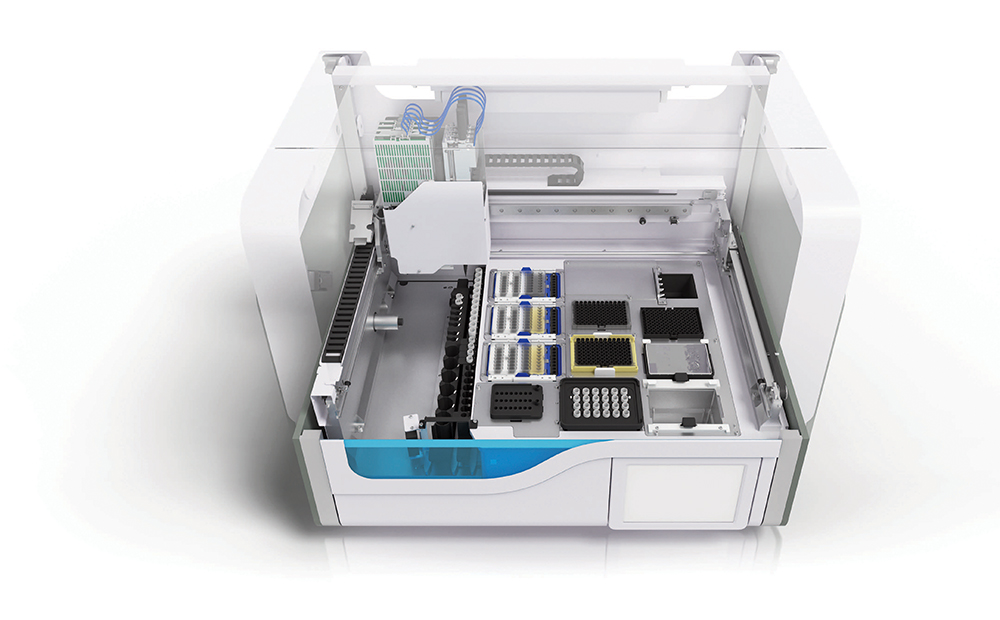
The Roche cobas 8800 can test 4,128 swab samples a day. That’s possible because human interaction is only required for three steps — the loading of reagents and other test consumables; the racking and insertion of trays containing RFID tracked or barcoded samples into another segment of the system; and then the emptying of a waste bin and reading of digitized results that are sent onward.
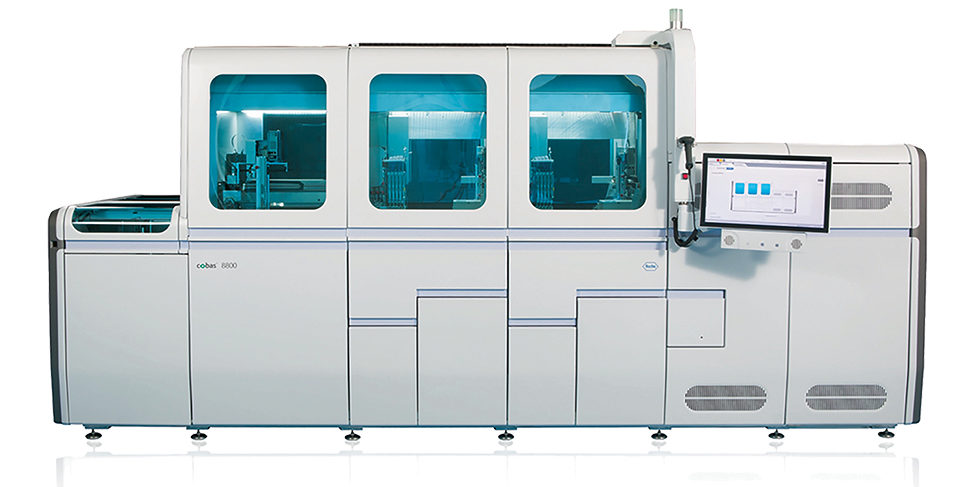
The actual test executed by the Roche cobas compares nucleic acids from patient mucus or saliva to sequences found in known coronavirus strains.


An X-Y-Z Cartesian gantry uses a precision gripper to pick test tubes containing patient samples out of loaded racks and place those tubes on a specialized conveyor for single-file buffering. The tubes get grouped by another robot and put into an automatic centrifuge module; a precision destopper pulls the tops off the test tubes to allow sample access. Sample tubes go through a high-speed labeling module rivaling the fastest labelers in the packaging industry to get stickered with secondary barcoding. Then they go through an aliquoter — a specimen-sampling unit that takes the exact amount of sample required for repeatable testing.
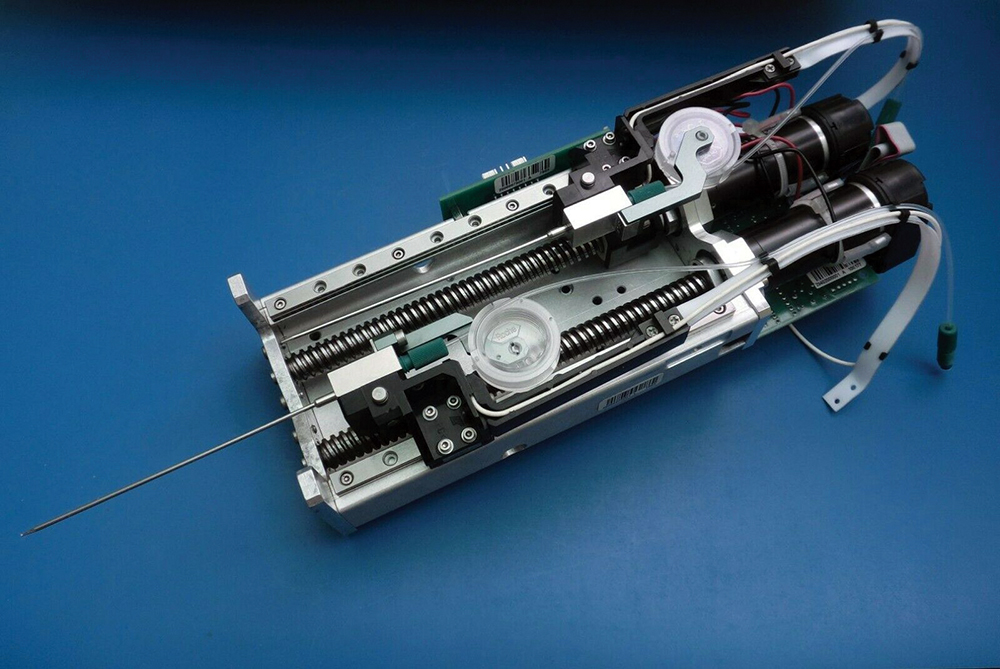
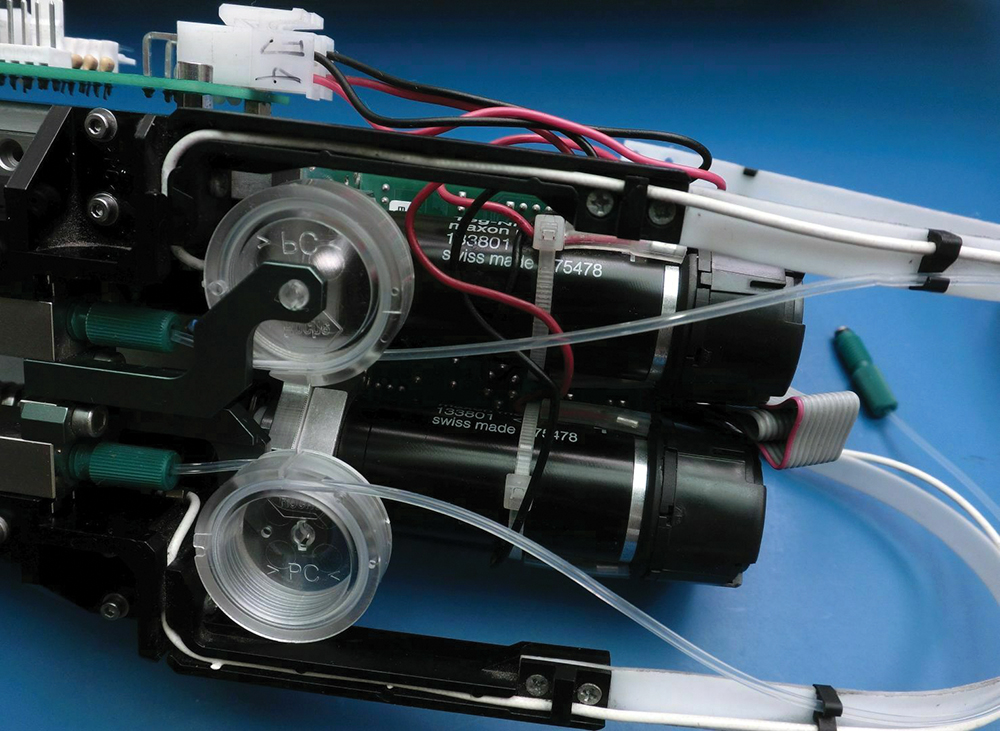
In fact, we’ve covered AutoSorter 1200 technology from the Motoman Robotics Division of Yaskawa America for similar aliquoter systems on high-speed specimen processing equipment.
Another Cartesian gantry takes the end-effector arrays to a station to receive disposable culture tubes and (after they’ve been used to sample specimens) release them into a waste chute. Networks of tiny precision conveyors take tubes to additional stations and then to a restopper machine unit and finally to an output buffer rack.
Test results are automatically sent to laboratory information system (LIS) and electronic medical record (EMR) networks and in some cases can be displayed locally on an HMI.
For more information on the Roche cobas series, visit diagnostics.roche.com.
In another design related to manufacturing testing devices, Roche is using an eXtended Transport System from Beckhoff to manufacture cobas plasma separation cards (PSCs) that simplify and improve the monitoring of patients with viral infections even if they are in remote locations. Read more about the XTS starting in our coverage of conveyors and linear-transport systems.
This article appears in the 2020 Design World Trends issue. Read other articles from this annual update here: 2020 Design World Motion Trends — the full URL library.






Leave a Reply
You must be logged in to post a comment.